This Once Derided Technology Lets You See at an Atomic Scale
It started out as “blobology,” but this imaging technique earned three scientists a Nobel Prize.
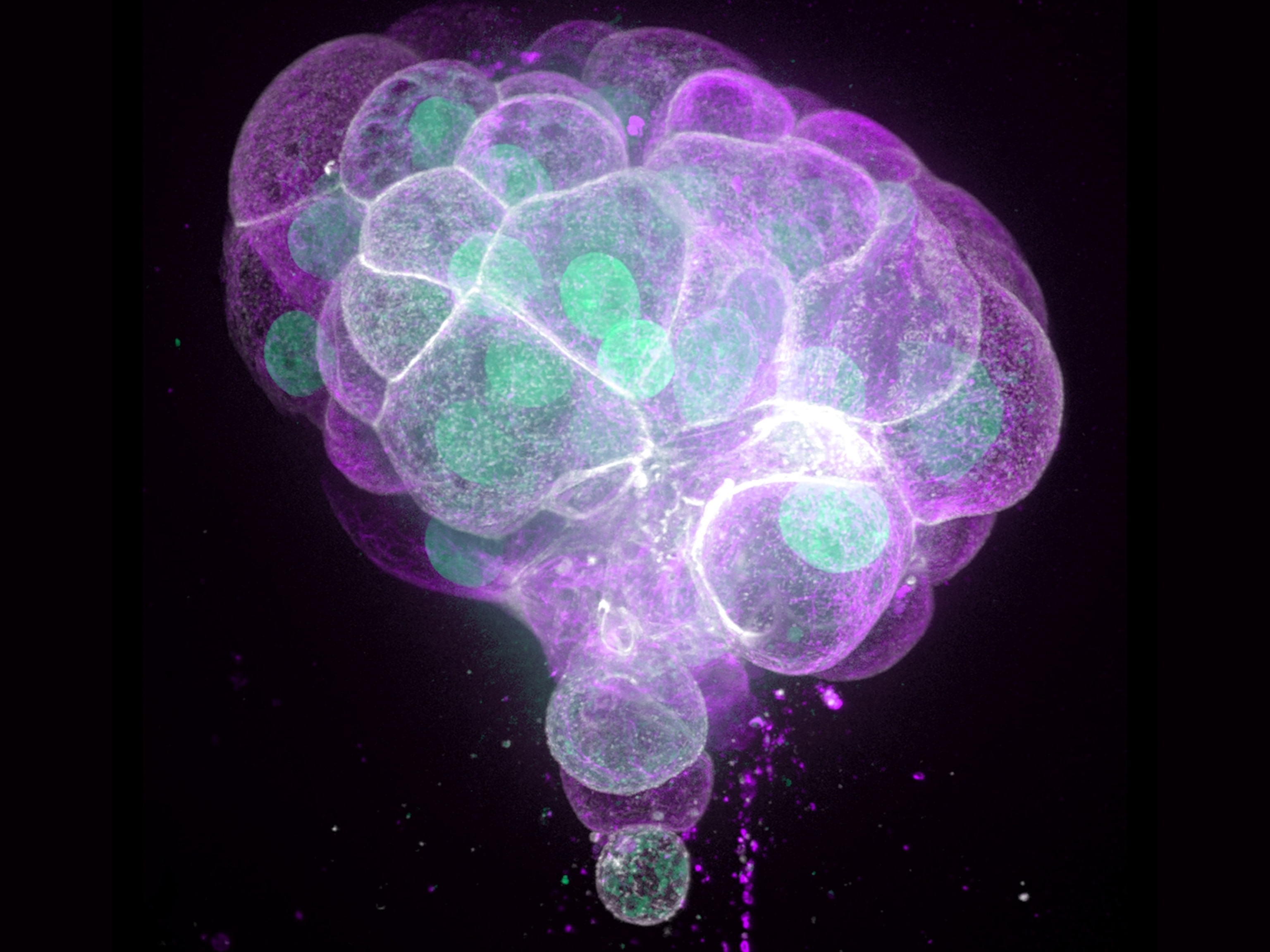
The kaleidoscopic ball above is more—and less—than meets the eye. This vivid terrain is actually a color-coded Zika virus, millions of which could fit in the period that ends this sentence.
We can see the virus here thanks to cryo-electron microscopy (cryo-EM), an extremely cool imaging technique that lets scientists visualize molecules, making it easier than ever to study life’s cellular machinery.
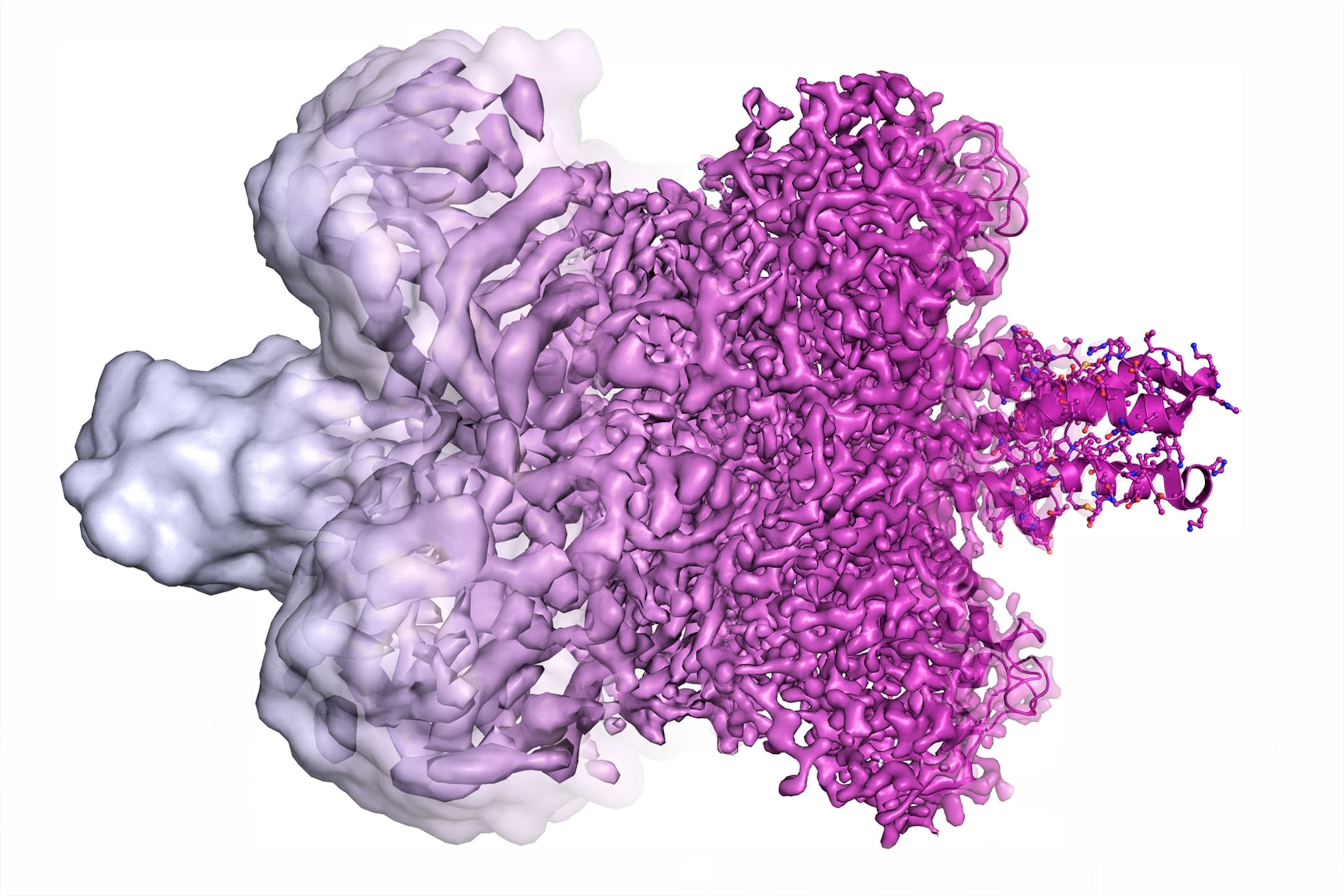
Cryo-EM works by firing an electron beam at a flash-frozen film of water containing copies of a given molecule. This “exposure” yields many 2-D images of the molecules at different angles, which algorithms then merge into one 3-D model. At first cryo-EM rendered molecules as vague lumps, so some dismissed it as “blobology” compared with x-ray crystallography, a less versatile high-resolution imaging technique. But in 2013 cryo-EM achieved atomic resolution for the first time.
“It wasn’t quite clear to me that we would get to atomic resolution; even 10 years ago I was sort of skeptical,” says Columbia University biophysicist Joachim Frank, who shares the 2017 Nobel Prize in chemistry for helping develop cryo-EM. Now proteins’ building blocks can be seen like beads on a string.
Cryo-EM allows us to see the proteins that stud our cells’ membranes—or even “film” drug molecules as they bind to their targets. Who knows where this biochemistry revolution will go next? “I’m very excited,” says Frank.