New research shows how genes can identify risks of breast cancer
Analysis of thousands of tumors shows that inherited genes determine how a breast tumor develops from its earliest stages.
A woman’s inherited genes are a powerful indicator of what kind of breast cancer she might develop, a recent study reveals.
Specifically, the genes dictate the visibility of epitopes, or proteins that decorate the outside of all cells, including cancerous ones. These epitopes function like a sign outside a business that identifies it as a bakery, law firm, or theatre. Conspicuous epitopes act like a flashing neon sign to attract the attention of patrolling immune cells.
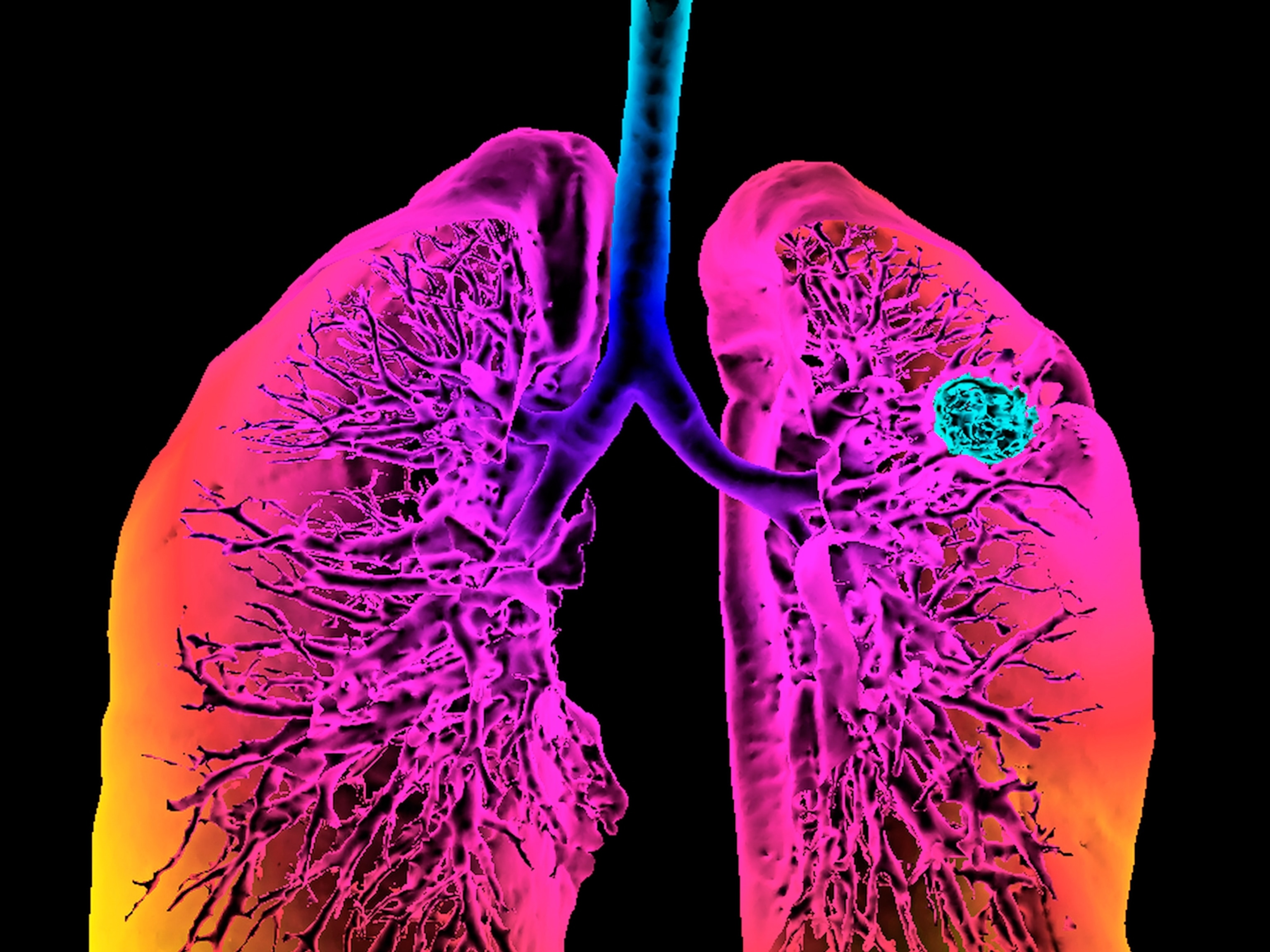
Using data from tens of thousands of patients, research published in Science shows cancers that had flashier epitopes and were detected by the immune system were less likely to become invasive. However, if those flashy cancer cells escaped immune detection, they had a higher chance of becoming metastatic.
These results indicate that a tumor’s deadly potential is established very early during disease, says lead author Christina Curtis, a Stanford University cancer researcher and data scientist. In the future, this could help women more at risk of aggressive cancer (i.e. those whose immune systems might miss flashy signs) get increased screening and novel therapeutics—thereby saving lives.
“If we can understand which mutations are driving the development of these more aggressive tumors, it will be extremely helpful for predicting which patients are going to suffer more from their disease,” says Michalina Janiszewska, a cancer biologist at the University of Florida Scripps Institute.
How our immune system identifies cancer
A gene mutation causing cells to go rogue isn’t as rare as you might think, says Kornelia Polyak, a molecular biologist at the Dana-Farber Cancer Institute in Boston. That’s why our immune systems are constantly surveilling for anomalies—and are generally very effective at detecting them. Our immune systems scan all cells’ epitopes, the array of proteins that decorate a cell’s exterior, which serves as a molecular identity card.
Genetics play an important role in this as genes influence which types of signs your cells display. Conspicuous epitopes attract the immune system’s attention, leading to the cell’s destruction.
Curtis’ study shows even before cancer cells form a recognizable tumor, they display epitopes that can mark their malignant potential.
Cancer cells that are ‘born to be bad’
More than a decade ago, scientists believed that most cancer was something that happened over the course of a lifetime. The thought was that healthy cells produce cancerous ones randomly, and whether the cancer turned invasive and deadly was also up to chance—and it was impossible to pass on to children.
Then, in 2015, Curtis found evidence that genetics affect the epitopes of all cells, including cancer. Using samples of colorectal tumors, Curtis found that even at their earliest stages, aggressive, metastatic colorectal tumors looked different on a cellular level than their more indolent counterparts.
Not only can young cancer cells look different from healthy cells—cancer cells often also look very different from each other. Polyak’s work showed high levels of molecular and genetic variation within a single breast tumor in an individual woman. Tumor cells were not identical clones of each other but could vary widely both in the genes they carried and their physical characteristics. This means that treatment like hormone therapy wouldn’t necessarily wipe out tumor cells: some cells can survive and seed a fully resistant, triple negative breast tumor that is more aggressive and has few treatment options, Polyak says.
Breast cancer forecasts
Scientists have known about the importance of inherited mutations in genes like BRCA1 and BRCA2 in impacting cancer development since the 1980s, Curtis says. Individuals carrying certain versions of these two genes have an extremely high risk of developing breast, ovarian, and other cancers. But many individuals who have inherited an increased risk for breast cancer don’t have these two variants, leaving scientists to ask where this risk comes from.
What Curtis and her postdoctoral fellow Kathleen Houlahan wanted to know was how inherited immune differences could help explain why some women develop breast cancers that were readily treatable, and others relapse decades after seemingly successful treatment.
This type of investigation required lots of data, so Houlihan and Curtis stitched together information from a range of datasets, including tumor cell atlases and cancer registries. Their analysis of over 6,000 breast tumors showed that cells varied in what scientists call their “germline epitope burden,” which could help predict how aggressive a cancer might form.
What they found is that that women with higher germline epitope burdens (i.e. more flashy neon signs) were more likely to catch and eliminate cancer cells early and prevent it from becoming invasive. However, if women had cancer with flashy signs and escaped immune surveillance, "the pattern flips," Houlahan said in a report for Stanford University. The cancer would be more likely to become aggressive, learning to outsmart the the immune system. It could become invasive, affecting nearby tissue, or spread and metastasize to distant parts of the body.
The future of breast cancer prevention
This study, Polyak said, could help separate women into high- and low-risk groups, which is important for prevention. Women who were less able to detect flashy cancer cells could be identified and receive more frequent mammograms and other screenings. “This paper is just the tip of the iceberg,” she says. “The more we know, the better we can predict risk.”
Curtis says that these results are important not just in advancing our understanding of how cancer develops but also in creating better precision medicine treatments for breast and other cancers. She envisions a potential genetic test that can help predict those at the highest risk of developing aggressive breast cancer and developing therapies for these specific subtypes of disease.
“Right now, our surveillance is a bit haphazard,” Curtis says. “We’re not doing enough for the women at highest risk for dying if we don’t treat them and surveil them differently. I think we’re failing.”