First Human Embryos 'Edited' in U.S.—Get the Facts
The work, which removed a gene mutation linked to a heart condition, is fueling debate over the controversial tool known as CRISPR.
What if you could remove a potentially fatal gene mutation from your child’s DNA before the baby is even born? In an advance that's as likely to raise eyebrows as it is to save lives, scientists just took a big step toward making that possible.
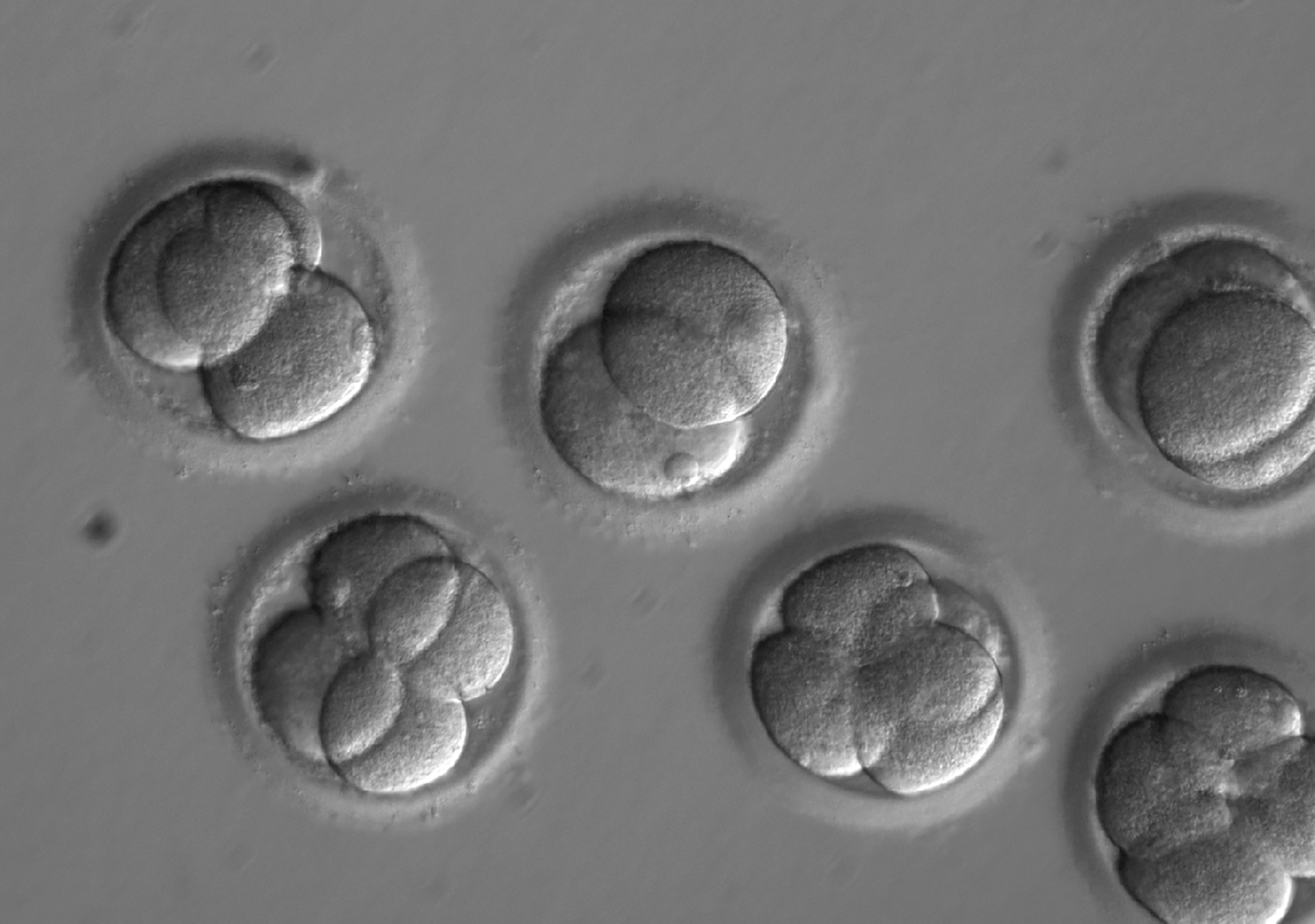
For the first time, researchers in the United States have used gene editing in human embryos. As they describe today in the journal Nature, the team used “genetic scissors” called CRISPR-Cas9 to target and remove a mutation associated with hypertrophic cardiomyopathy, a common inherited heart disease, in 42 embryos.
If CRISPR sounds familiar, there’s a reason: Ever since its introduction, it’s been at the center of a heated debate about the ethics of gene editing. (Read more about how the DNA revolution is changing us in National Geographic magazine.)
Scientists who want to explore the technique hail it as a biomedical advance that could one day give people the option not to pass down heritable diseases. The tool could also reduce the number of embryos that are discarded during fertility treatments because of worrisome genetic mutations.
But critics counter that it takes more than safety or even efficacy to make a procedure ethical.
“The scientists are out of control,” says George Annas, director of the Center for Health Law, Ethics & Human Rights at the Boston University School of Public Health, who thinks that scientists should not edit the genomes of human embryos for any reason. “They want to control nature, but they can't control themselves.”
(Take our poll: Should gene editing be used on human embryos?)
Healing Hearts
According to the Centers for Disease Control and Prevention, hypertrophic cardiomyopathy occurs in about one in 500 people. The condition causes the heart muscle to thicken and can lead to sudden cardiac arrest.
It takes only one gene mutation to cause the condition, and you can get the disease even if only one of your parents has the mutated gene. If you inherit it, there’s a 50 percent chance you will pass it on to your children.
For their work, Shoukhrat Mitalipov, principal investigator at the Oregon Health and Science University’s Center for Embryonic Cell and Gene Therapy, and his colleagues targeted the genetic mutations that cause the majority of hypertrophic cardiomyopathy cases.
First, they created 58 human embryos from the sperm of a male donor with the mutation and the egg of a female without the mutation. Then, they used CRISPR to cut the mutation out of the gene. The gene editing tool guides an enzyme called Cas-9 to a targeted position on DNA and snips the molecule at just the right spot. When things go right, the DNA repairs itself and the mutation disappears.
The technique isn’t always successful. In previous studies, some CRISPR-edited embryos developed mosaicism, a condition in which some cells have the unwanted mutations and others don’t.
So the team developed a new method: injecting sperm and CRISPR into the egg simultaneously instead of waiting until after fertilization to edit the genes. This time, they say, mosaicism did not occur.
All in all, the team was able to repair the gene mutation in about 70 percent of the embryos, and the study showed no unwanted changes at other sites in the edited DNA.
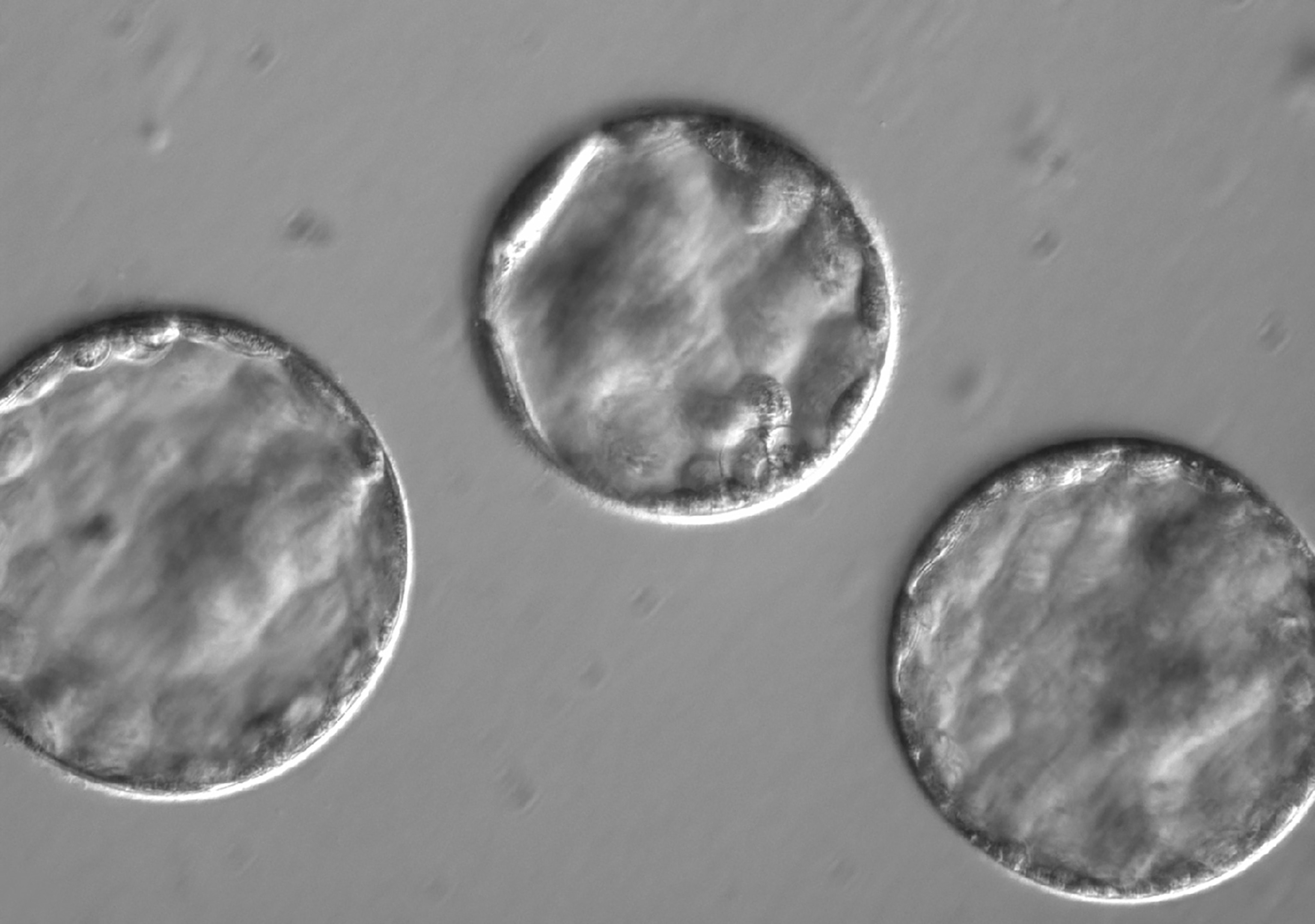
The team allowed the fertilized cells to develop into blastocysts—the stage at which embryos are usually implanted into the mother during fertility treatments. They showed normal development, the team reports. Then, the embryos were destroyed.
Science in Motion
“Of course further research and ethical discussions are necessary before proceeding to clinical trials,” study coauthor Paula Amato, adjunct associate professor of obstetrics and gynecology at OHSU, said during a press briefing on August 1.
Earlier this year, the National Academy of Sciences and National Academy of Medicine asked an international committee of scientists and ethicists to weigh in on the benefits and risks of genome editing in humans. (Find out why scientists think gene editing is both terrifying and terrific.)
The panel recommended that in the case of the human germline—genes passed down from generation to generation—scientists refrain from editing genes for any purpose other than treating or preventing a disease or disability. The report also insisted on a more robust public debate before such experiments begin.
In the United States, there’s currently a ban on using taxpayer funds for any research that destroys human embryos.
In this case, the researchers forged ahead with their work and used institutional and private funds. If the team can’t move ahead as quickly as desired in the U.S., they’ll consider pursuing their research in other countries.
While editing out genetic diseases may still seem far off, people should pay close attention to this kind of research, says Sakthivel Sadayappan, director of the heart branch of the University of Cincinnati’s Heart, Lung and Vascular Institute.
“This is exciting,” says Sadayappan, who was not involved in the new CRISPR study. “This is the future.”
The experiment’s small sample size certainly leaves something to be desired. But Sadayappan says it’s worth conducting and supporting the research. “Of course feasibility studies have issues,” he says. “But it’s the only way science can evolve.”
For Sadayappan, who researches hypertrophic cardiomyopathy, the stakes are high. Patients who have inherited the mutation from both parents, he says, “have no option other than this technology if they want to have offspring.”
Debating the Future
It’s already possible to screen for genetic defects within embryos during in vitro fertilization using a process called preimplantation genetic diagnosis. The team thinks their CRISPR technique could eventually be applied to gene mutations associated with other diseases, like cystic fibrosis.
In their paper, the team writes that their method may one day “rescue mutant embryos, increase the number of embryos available for transfer and ultimately improve pregnancy rates.”
“That’s just absurd,” says Annas. “They admit right up front that if you want to avoid having a baby with [the mutation], you can just not implant the embryos that are affected.”
Mitalipov disagrees: “Discarding half the embryos is morally wrong,” he tells National Geographic. “We need to be more proactive.”
Either way, Annas says, it’s time to revisit the conversation about how to regulate CRISPR in the United States. “My guess is that the regulators will be horrified.”
But for Mitalipov, the debate is a chance to inform the world about the technique’s potential. And for a scientist who has also cloned monkey embryos and even cloned human embryos to make stem cells, he knows plenty about how to ignite public debate.
“We’ll push the boundaries,” he says.